Energetix | Emily Greene-Hartsfield, ND
Most healthcare practitioners include sleep habits as part of a general lifestyle optimization, but how often is it considered with the same clinical rigor as dietary protocols, endocrine balance, or mitochondrial function? Emerging research invites us to consider elevating sleep from a wellness recommendation to an important part of metabolic care.
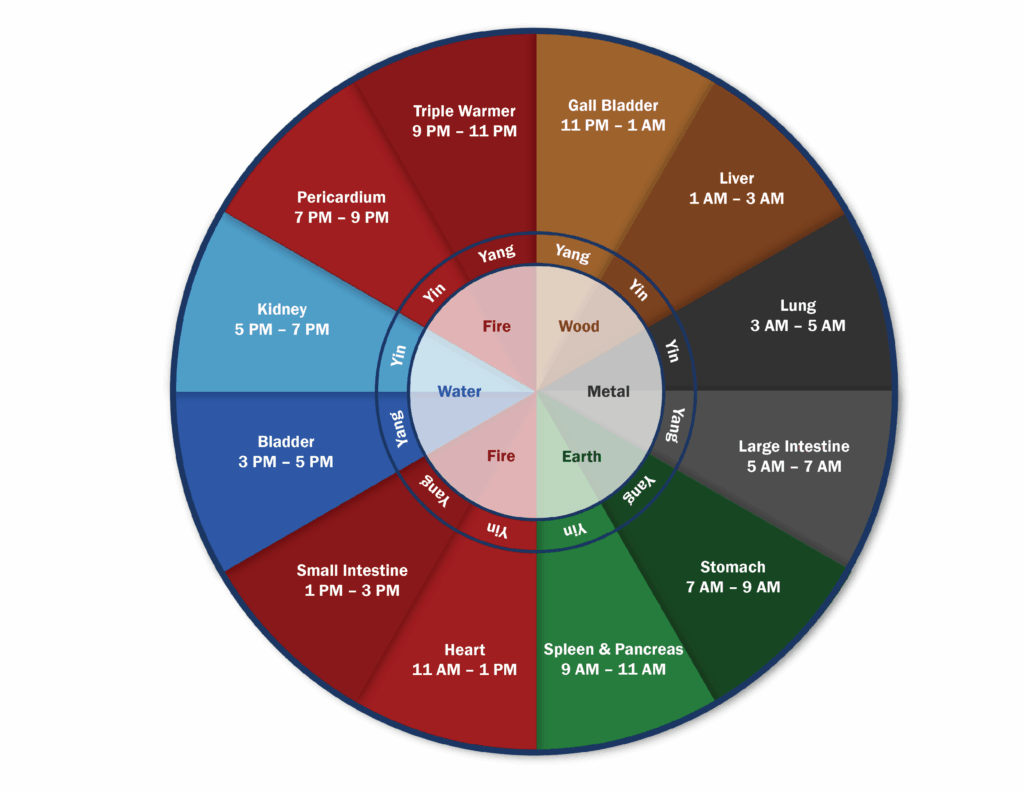
Circadian rhythm alignment, or the timing of biological rhythms, is more than a quality-of-life issue. It is a foundational regulator of insulin sensitivity, mitochondrial efficiency, hormone signaling, and the capacity for systemic repair. When circadian rhythm is compromised, so is metabolic resilience [1].
Circadian Rhythms: Metabolism's Master Clock
The circadian rhythm is an innate 24-hour cycle, governed by the suprachiasmatic nucleus (SCN) in the hypothalamus, that responds to light and plays a central role in regulating both sleep and metabolic processes. The SCN serves as the body’s master clock, synchronizing peripheral biological clocks by integrating inputs such as light exposure, hormone secretion, eating patterns, and physical activity.
These peripheral clocks exist in major organs and tissues, such as the liver, pancreas, fat, digestive tract, and muscles. These help to regulate vital metabolic functions, including:
- Glucose production and bile processing in the liver
- Insulin release and blood sugar response by the pancreas
- Breakdown of fat for energy and heat generation
- Movement of food through the digestive system
- Daily rhythms of gut bacteria
When sleep patterns are irregular, or meals occur late at night, or there is excessive light exposure after dark, the body’s internal timing can be disrupted. This misalignment in circadian rhythms can lead to problems such as insulin resistance, cholesterol imbalance, and weight gain, even in patients who maintain a healthy diet and stay physically active. [2].
“Sleep is not simply rest; it’s a metabolic reset. Circadian rhythm alignment governs everything from insulin sensitivity to mitochondrial output. When we restore rhythm, we restore resilience.” – Emily Hartsfield, ND
Hormonal Entrainment During Sleep
Sleep influences metabolism through hormonal entrainment. This is the synchronization of hormone secretion with external and behavioral cues, such as light exposure, sleep-wake patterns, timing of meals, and physical activity.
During sleep, especially in slow-wave (non-REM) stages, the body recalibrates several metabolic hormones:
- Insulin: During early sleep stages, insulin sensitivity tends to be lower. Sleep restriction or sleep fragmentation makes it more difficult for the body to respond to insulin, causing the liver to release more sugar into the bloodstream. [3]
- Leptin: This hormone signals satiety, and naturally rises during sleep to help regulate appetite and energy balance. In contrast, ghrelin, which promotes hunger, is suppressed. Disturbed sleep can promote overeating and weight gain. [4]
- Cortisol: Typically high in the early morning, this hormone declines by the evening. Stress or circadian rhythm misalignments can cause cortisol levels to stay high at night, which may lead to abdominal (belly) fat gain and blood sugar concerns. [5]
Clinical studies have shown that even one night of partial sleep deprivation can decrease insulin sensitivity by up to 25%, which is comparable to the effect of a high-fat diet. [6]
Mitochondrial Timing and Function
Mitochondrial health is essential to metabolic function. These energy centers help to regulate glucose and fat metabolism following circadian rhythms that influence their activity throughout the day. The ability of the mitochondria to produce energy, make repairs, and eliminate unhealthy parts varies depending on the time of day. Disrupted sleep alters these dynamics, leading to:
- Less ATP (energy production)
- More Oxidative stress from excess free radicals
- Difficulty burning fat efficiently for fuel
The Gut-Microbiome Clock
When the body’s internal clock is out of sync, the balance of gut bacteria is disrupted, weakening the digestive lining and triggering inflammation.
The internal environment of the digestive tract influences metabolic and immune function, which is also governed by circadian rhythms. This plays a crucial role in regulating blood sugar and inflammation.
For practitioners working with complex metabolic inflammation, this is an important and often missed opportunity to support healing.
While individualized protocols will vary, general guidance includes:
– Encouraging sleep and wake consistency
– Reducing artificial light exposure after sunset
– Timing meals to align with daylight hours
– Utilizing stress management techniques that help bring cortisol levels back to a healthy daily rhythm (high in the morning and low at night)
Clinical Support for Circadian Rhythm Restoration
Here are some effective solutions that work with the body’s natural rhythms to support its internal timing and help to restore balance:
Homeopathic Remedies: Consider formulas that help reset sleep-wake patterns and address autonomic nervous system regulation. Also consider remedies that support the hypothalamus.
- Hypothalmapath®: For symptoms related to endocrine and nervous system function.
Spagyric Botanical Formulas:
- Core Valerian: Supports the nervous system and promotes sleep without interfering with the deep, restorative stages of sleep.
- Core Licro Blend: Supports cortisol metabolism by slowing its breakdown, which can be beneficial for patients with low morning cortisol levels.
- Core Ashwagandha: Supports the healthy function of the stress response system (HPA axis). Clinical data indicate that it lowers evening cortisol and enhances sleep quality [11].
Nutritional Formulas
- Seratran: Designed to support healthy serotonin levels and mood balance.
- Melatonin Spray: Supports healthy sleep patterns which helps maintain mood balance and memory function.
Practitioners who integrate circadian rhythm understanding into their diagnostic and therapeutic approach can uncover previously overlooked drivers of dysregulation and provide patients with more sustainable strategies for recovery.
[1] Cedernaes, J., Waldeck, N., & Bass, J. (2019). Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Nature Neuroscience, 22(4), 589–596. https://pages.ucsd.edu/~mboyle/COGS163/pdf-files/Circadian%20clocks%20and%20insulin%20resistance-2018.pdf
[2] Fatima, N., & Rana, S. (2020). Metabolic implications of circadian disruption. Pflügers Archiv – European Journal of Physiology, 472(4), 513–526. https://doi.org/10.1007/s00424-020-02381-6
[3] Brown, P. (2020). Sleep: How quality and duration affect insulin sensitivity and glucose control. Diabetes & Primary Care, 22(4), 129–130. https://diabetesonthenet.com/diabetes-primary-care/sleep-how-quality-and-duration-affects-insulin-sensitivity-and-glucose-control/
[4] Spiegel, K., Tasali, E., Penev, P., & Van Cauter, E. (2004). Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine, 141(11), 846–850. https://doi.org/10.7326/0003-4819-141-11-200412070-00008
[5] Wright, K. P., McHill, A. W., Birks, B. R., Griffin, B. R., Rusterholz, T., & Chinoy, E. D. (2013). Entrainment of the human circadian clock to the natural light-dark cycle. Current Biology, 23(16), 1554–1558. https://doi.org/10.1016/j.cub.2013.06.039
[6] Buxton, O. M., Pavlova, M., Reid, E. W., Wang, W., Simonson, D. C., & Adler, G. K. (2010). Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes, 59(9), 2126–2133. https://doi.org/10.2337/db09-0699
[7] Gao, Y., Gan, T., Jiang, L., Yu, L., & Tang, D. (2020). Association between shift work and risk of type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of observational studies. Chronobiology International, 37(1), 29–46. https://doi.org/10.1080/07420528.2019.1683005:contentReference[oaicite:10]{index=10}
[8] Shostak, A., Meyer-Kovac, J., & Oster, H. (2013). Circadian regulation of adipose function. Adipocyte, 2(4), 201–206. https://doi.org/10.4161/adip.24733
[9] Hermida, R. C., Ayala, D. E., Mojón, A., & Fernández, J. R. (2013). Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiology International, 30(1–2), 87–98. https://doi.org/10.3109/07420528.2012.708374
[10] Remchak, M.-M. E., Heiston, E. M., Ballantyne, A., Dotson, B. L., Stewart, N. R., Spaeth, A. M., & Malin, S. K. (2022). Insulin Sensitivity and Metabolic Flexibility Parallel Plasma TCA Levels in Early Chronotype With Metabolic Syndrome. The Journal of Clinical Endocrinology & Metabolism, 107(8), e3487–e3496. https://doi.org/10.1210/clinem/dgac233
[11] Haber, M., Czachor, A., Kula, P., Juśkiewicz, A., Grelewicz, O., Kucy, N., Servaas, E., Kotula, A., & Siemiątkowski, R. (2024). Ashwagandha as an Adaptogen: Its Influence on Sleep Patterns, Stress Response, and Anxiety in Modern Life. Journal of Education, Health and Sport, 68. https://doi.org/10.12775/JEHS.2024.68.5532




